The amount of energy remains constant and energy is neither created nor destroyed. Energy can be converted from one form to another (potential energy can be converted to kinetic energy) but the total energy within the domain remains fixed.
If you take any volume of space, then the total energy inside that volume at a given time is always the amount that was there earlier, plus the total amount that has come in through the surface, minus the total amount that has gone out through the surface.
Albert Einstein's theory of relativity shows that energy and mass are the same thing, and that neither one appears without the other. Thus in closed systems, both mass and energy are conserved separately.
The new feature of relativistic physics is that "matter" particles (such as those constituting atoms) could be converted to non-matter forms of energy, such as light; or kinetic and potential energy (example: heat).
However, this conversion does not affect the total mass of systems, since the latter forms of non-matter energy still retain their mass through any such conversion.
Examples of Conversion of Energy:

For instance, a coal-fired power plant involves these power transfers:
1. Chemical energy in the coal converted to thermal energy
2. Thermal energy converted to kinetic energy in steam
3. Kinetic energy converted to mechanical energy in the turbine
4. Mechanical energy of the turbine converted to electrical energy, which is the ultimate output
The motion of a pendulum is a classic example of mechanical energy conservation. A pendulum consists of a mass (known as a bob) attached by a string to a pivot point.
As the pendulum moves it sweeps out a circular arc, moving back and forth in a periodic fashion. Neglecting air resistance (which would indeed be small for an aerodynamically shaped bob), there are only two forces acting upon the pendulum bob.
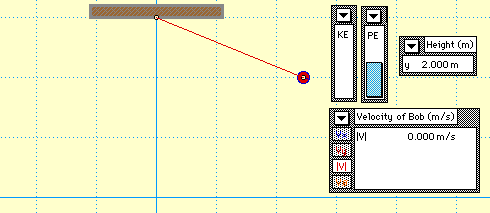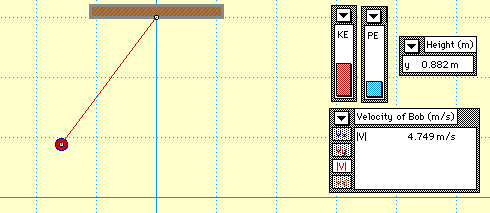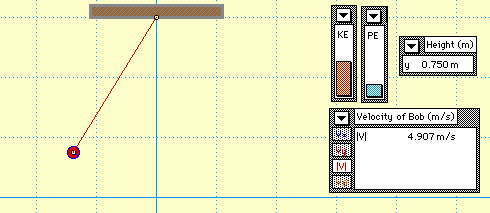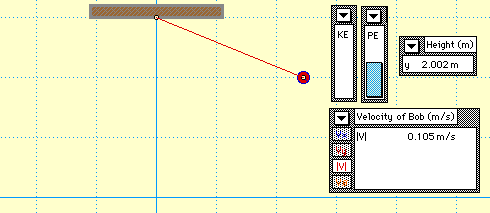
In this animation, you see a mass attached to the end of a string which forms a pendulum. The pendulum begins with only gravitational potential energy (GPE) since it is not moving yet. After being released, GPE is turned into kinetic energy (KE). Notice that no matter where the pendulum is, the sum of the GPE and KE is always equal to the original amount of energy the system started with. This demonstrates the Law of Conservation of Energy.
Energy is Conserved)
Questions:
* Where does the pendulum have the highest velocity?
* How does the original height of the pendulum compare to its final height?
This Animation and illustration shows an ideal situatuation.However, using our common sense we know that it's impossible for the pendulum to swing higher than the
height h without giving it a push yourself. If there was no friction, the pendulum would swing back and forth forever because of the law of conservation of energy.
In reality, we know that eventually the pendulum comes to a stop.This is due to the frictional forces.As the pendulum swings, some of its total energy is converted to thermal energy due to frictional forces and dissipated to the surroundings and cannot be converted back to the kinetic or gravitational energy.The thermal energy must have come from the original gain in gravitational potential energy.
Hence, as a result , the pendulum bob cannot attain its initial height. It continues to lose its energy.When all its original gain in potential energy has been converted to thermal energy, the pendulum bob stops moving.
Efficiency:
We all use devices every day that use energy - or more accurately, transfer energy from one form to another. Everything we use wastes energy - some of the energy transfers into forms that are not useful to us.
Very few devices can transfer energy from one form into another without wasting some on the way.
For Example, A light bulb is designed to turn electrical energy into light energy. But most bulbs produce a lot of heat energy too. That energy has not been lost but it has been wasted.
To measure the efficiency of a device, calculate what percentage of the total energy put in, became useful output energy.

For example: a bulb is provided with 100J of electrical energy but only produces 20J of light. The fraction turned into light is 20J out of 100J = 20/100.

In percentages, that's 20/100 x 100% = 20%.
So the equation for efficiency is:
efficiency (%) = (useful energy out ÷ total energy in) x 100.
Efficiency is normally calculated as a percentage - something 90% efficient is considered good at its job. Devices that transfer only 5% of the energy they use into something useful are inefficient (very wasteful).
When energy is transferred,some of the energy turns into forms we don't want.
This energy is called wasted energy.
Wasted energy takes the form of heat and sometimes sound or light.
During any energy transfer, some energy is changed into heat.The heat becomes spread out into the environment.
This dispersed energy becomes increasingly difficult to use in future energy transfers.In the end, all energy is transferred into heat.
Efficiency is not the same as cost-effectiveness.
No comments:
Post a Comment