There are five main states of matter. Solids, liquids, gases, plasmas, and Bose-Einstein condensates are all different states of matter. Each of these states is also known as a phase.
Elements and compounds can move from one phase to another phase when special physical forces are present. One example of those forces is temperature. The phase or state of matter can change when the temperature changes. Generally, as the temperature rises, matter moves to a more active state.
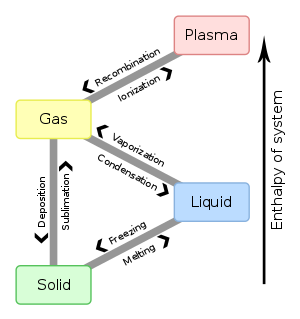
Phase describes a physical state of matter. The key word to notice is physical. Things only move from one phase to another by physical means. If energy is added (like increasing the temperature or increasing pressure) or if energy is taken away (like freezing something or decreasing pressure) you have created a physical change.

| PROPERTY | SOLID | LIQUID | GAS |
| shape | fixed | same as container (indefinite) | same as container (indefinite) |
| volume | definite | definite | fills entire container (indefinite) |
| ability to flow | no | yes | yes |
| can be compressed | very slightly | very slightly | yes |
| volume change with heating | very small | small | large |
| Density | High | High | Low |
|
|
|
|
|
One compound or element can move from phase to phase, but still be the same substance. You can see water vapor over a boiling pot of water. That vapor (or gas) can condense and become a drop of water. If you put that drop in the freezer, it would become a solid. No matter what phase it was in, it was always water. It always had the same chemical properties. On the other hand, a chemical change would change the way the water acted, eventually making it not water, but something completely new

No comments:
Post a Comment