Heat Capacity
What is Internal Energy?
Internal energy is defined as the energy associated with the random, disordered motion of molecules. It is separated in scale from the macroscopic ordered energy associated with moving objects; it refers to the invisible microscopic energy on the atomic and molecular scale.
For example, a room temperature glass of water sitting on a table has no apparent energy, either potential or kinetic . But on the microscopic scale it is a seething mass of high speed molecules traveling at hundreds of meters per second. If the water were tossed across the room, this microscopic energy would not necessarily be changed when we superimpose an ordered large scale motion on the water as a whole.
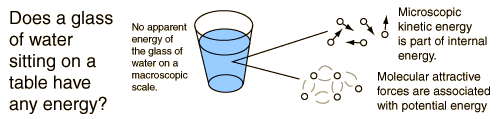
If the temperature of a substance rises, it is due to an increase in the average kinetic energy of its particles only.
What is heat capacity?
Heat capacity (usually denoted by a capital C, often with subscripts) is the measurable physical quantity that characterizes the amount of heat required to raise a body's temperature by 1K or 1*C.
Amount of Thermal energy needed depends on Object's mass.
Equation for Heat Capacity:

where,
Q - thermal energy needed in Joules(J)
T - Temperature change in K or *C
The unit for Heat Capacity(C) is J/Kg
Specific Heat Capacity
Specific Heat Capacity (c) of a substance is the amount of heat required to raise the temperature of 1g of the substance by 1oC (or by 1 K).
The units of specific heat capacity are J oC-1 g-1 or J K-1 g-1
Equation for Specific Heat Capacity:

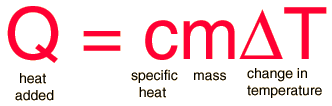
where,
Q= Heat supplied to substance,
m= Mass of the substance,
c= Specific heat capacity,
T= Temperature rise.
No comments:
Post a Comment