Phase Transition of Water
Melting and Solidification
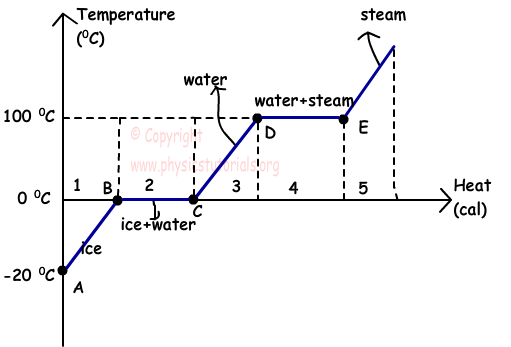
This graph shows the phase diagram of water.
At the beginning we have ice at -20 ºC. Ice gain heat in the interval of points A and B, and its temperature becomes 0 ºC that is the melting point of it. We have only ice in the 1st region.
As you can see between the points B and C, temperature of the mass does not change, because its state is changing in this interval. Gained heat is absorbed and spent on breaking the bonds of molecules.
2nd region includes both water and ice. .
After melting process completed, in the 3rd region there is only water and temperature of water starts to increase. When the temperature of the water becomes 100 ºC, it starts to boil and evaporation of it speeds up.
In region 4 our mass exists in two state, water and steam. After completion of evaporation, all water converted to the steam and in region 5 we have only vapor of water.
The graph given below shows the relation of temperature vs. heat of water vapor that lost heat.
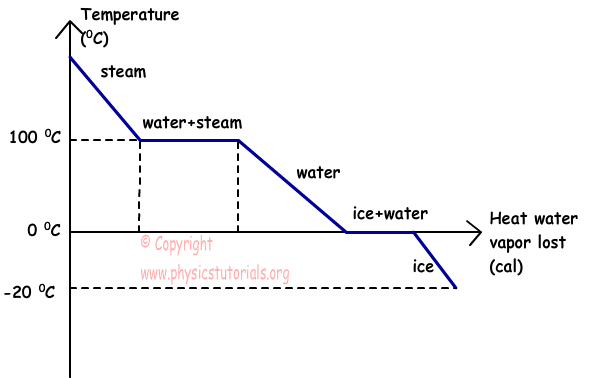
This graph shows the condensation of water vapor which lost heat.
As it seen from the graph, steam lost heat and its temperature decreases at 100 ºC, at this temperature it condensate and becomes water, after heat lost it reaches at temperature 0 ºC and starts to freeze. Finally, if it continues to lost heat, their temperature also continues to decrease.
Evaporation

Evaporation is a type of vaporization of a liquid, that occurs only on the surface of a liquid.
Evaporation is a type of phase transition; it is the process by which molecules in a liquid state (e.g. water) spontaneously become gaseous (e.g. water vapor). Generally, evaporation can be seen by the gradual disappearance of a liquid from a substance when exposed to a significant volume of gas. Vaporization and evaporation however, are not entirely the same processes.
Evaporation requires thermal energy from the surroundings.Thermal energy from our bodies helps the water on our skin to evaporate taking away thermal energy from the surface of your skin helping us cool down.
Evaporation
1.Occurs at any temperature
2.Slow Process
3.Takes place only at the liquid surface
4.No bubbles are formed in the liquid
5.Temperature may change
6.Thermal energy is supplied by the surroundings
Boiling
1.Occurs at fixed temperature
2.Quick Process
3.Takes place throughout the liquid
4.Bubbles are formed in the liquid
5.Temperature remains constant
6.Thermal energy is supplied by an energy source

How does evaporation take place?
We know that the molecules are never at rest. They can have slight translational and vibrational motions about their mean positions.
They thus posses some amount of kinetic energy. Sometimes the molecules collide with each other and exchange their kinetic energies. Thus at any given time, the kinetic energy of a few molecules may become quite large and they can escape from the surface. The probability of escape from the surface is larger for molecule 1, as its cohesive force holding it back to the liquid is less than that experienced by molecule 2. This is how evaporation takes place.

Factors affecting Rate of Evaporation
Temperature
The rate of evaporation increases as the temperature of a liquid is increased, as it is an endothermic process. For example, a glass of hot water evaporates more rapidly than a glass of cold water.
Surface area
The larger the exposed surface area of the liquid the greater is the number of molecules escaping from its surface.
Humidity of Surrounding Air
If the air already has a high concentration of the substance evaporating, then the given substance will evaporate more slowly.
If the air is already saturated with other substances, it can have a lower capacity for the substance evaporating.
Flow rate of air
This is in part related to the concentration points above. If fresh air is moving over the substance all the time, then the concentration of the substance in the air is less likely to go up with time, thus encouraging faster evaporation. This is the result of the boundary layer at the evaporation surface decreasing with flow velocity, decreasing the diffusion distance in the stagnant layer.
Pressure
Evaporation happens faster if there is less exertion on the surface keeping the molecules from launching themselves.
Strength of intermolecular forces
The ease of evaporation of a liquid is related to the strength of the attractive forces between the molecules in the liquid. In polar liquids cohesive forces are strong while in non-polar liquids the cohesive forces are very weak and the molecules escape easily. For example, ether evaporates more rapidly than ethyl alcohol while ethyl alcohol evaporates quicker than water.
Presence of impurities
Impurities affect the vapour pressure of a liquid appreciably. The non-volatile impurities lower the vapour pressure of a liquid. If the liquid contains impurities, it will have a lower capacity for evaporation.
 where:
where: