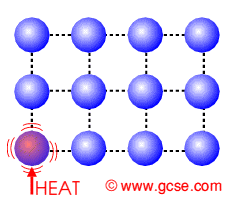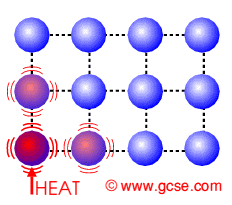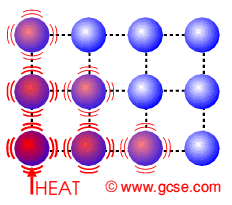
The centigrade temperature scale, more properly known as the Celsius temperature scale, is a scale for measuring temperatures which is based on the behavior of water at normal pressure. This scale is widely used in much of the world to express temperatures, with a few holdout nations using the Fahrenheit temperature scale. The Celsius scale is also used as the benchmark for the Kelvin scale, which is used in the scientific community.
Fixed Points

1. Ice Point(0*C) - Temperature of pure melting point at one atmosphere
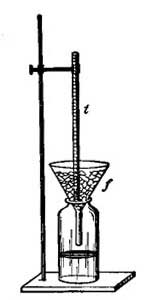
Calibration at the Ice Point of Water
1. Fill a 600 mL Beaker three-quarters full of crushed Ice.
2. Add enough pre-cooled distilled Water to cover the Ice, but not so much Water such thatthe Ice floats.
3. Thoroughly stir the Ice-Water mixture.
4. Insert the thermometer into a rubber stopper with a slit hole. (Be careful doing this!!!)
5. Use a ring stand and clamp to suspend the thermometer in the Ice-Water bath up to theImmersion Mark. (If you have a Total Immersion thermometer, see you laboratory
instructor for directions.)
6. Allow the temperature shown by the thermometer to stabilize. After 3 minutes at the stable temperature, record the temperature to the correct precision.
7. The Ice Point of Water is remarkably stable at 0.00oC.
2. Steam Point - Temperature of steam from water boiling at one atmosphere

Calibration at the Boiling Point of Water
1. Set up a hot plate with a 500 mL Florence Flask resting on it. The flask should be
supported by a clamp from a ring stand.
2. Fill the flask about half full with distilled Water. Add a few boiling chips to promote smooth boiling.
3. Clamp the thermometer to the ring stand as before such that the immersion mark is in the neck of the flask.
4. Turn on the hot plate and allow the Water to come to its Boiling Point.
5. Allow the temperature shown by the thermometer to stabilize. After 3 minutes at the stable temperature, record the temperature to the correct precision.
6. The Boiling Point of Water is extremely sensitive to the atmospheric pressure. Use the barometer to measure the atmospheric pressure. Use the data in the Appendix to determine the correct Boiling Point of Water.
*For Centigrade scale, the interval between the ice point and steam point is divided into 100 equal parts.Each mark on the thermometer is a measure of 1 *C.
General Equation for Centigrade Scale
Let 'x' be the physical property of the thermometric substance which varies continuously with temperature.Quantity of 'X' can vary according to the various physical property.(e.g. If X refers to the length of Mercury column in a Mercury-i-glass thermometer, the quantity of X will be cm or m)
Y*C = [(X(y)-X(0*C))/(X(100*C)-X(0*C))]x 100%
The Kelvin Scale
The Kelvin temperature scale (K) was developed by Lord Kelvin in the mid 1800s.The unit for this scale is Kelvin(K). The zero point on this scale is base on the point at which the pressure of all dilute gases mathematically project to zero from the triple point of water.The triple point is the temperature at which liquid water, ice, and water vapor can coexist simultaneously.
When a material is cooled, it looses heat, and its temperature decreases, until a point is reached where it has no more heat left to loose. At this point it is not possible to lower the temperature any further. This low temperature is called absolute zero. Lord Kelvin suggested that this absolute zero temperature be the basis of a new scale which begins with the value zero at absolute zero. Just like the Celsius scale, there is a difference of 100 degrees between the freezing and boiling points of water.(1*C = 1K)
Kelvin = degrees Celsius + 273