Sound is a mechanical wave that results from the back and forth vibration of the particles of the medium through which the sound wave is moving. If a sound wave is moving from left to right through air, then particles of air will be displaced both rightward and leftward as the energy of the sound wave passes through it. The motion of the particles is parallel (and anti-parallel) to the direction of the energy transport. This is what characterizes sound waves in air as longitudinal waves.

A vibrating tuning fork is capable of creating such a longitudinal wave. As the tines of the fork vibrate back and forth, they push on neighboring air particles. The forward motion of a tine pushes air molecules horizontally to the right and the backward retraction of the tine creates a low-pressure area allowing the air particles to move back to the left.
How is sound produced?
Sound is produced by anything that vibrates or changes air pressure rapidly. It could be a bell vibrating, a vocal cord, the sound board of a guitar, etc. The vibrations cause molecules of air to vibrate, and send the vibrations out in all directions. The vibrating air molecules reach your ear and cause your eardrum to vibrate. You perceive the vibrations as sound.
How does Sound Travel?
Sound is transmitted through gases, plasma, and liquids as longitudinal waves, also called compression waves. Through solids, however, it can be transmitted as both longitudinal waves and transverse waves.
Matter in the medium is periodically displaced by a sound wave, and thus oscillates. The energy carried by the sound wave converts back and forth between the potential energy of the extra compression (in case of longitudinal waves) or lateral displacement strain (in case of transverse waves) of the matter and the kinetic energy of the oscillations of the medium.
Because of the longitudinal motion of the air particles, there are regions in the air where the air particles are compressed together and other regions where the air particles are spread apart. These regions are known as compressions and rarefactions respectively. The compressions are regions of high air pressure while the rarefactions are regions of low air pressure. The diagram below depicts a sound wave created by a tuning fork and propagated through the air in an open tube. The compressions and rarefactions are labeled.
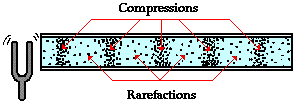
The wavelength of a wave is merely the distance that a disturbance travels along the medium in one complete wave cycle.A longitudinal wave consists of a repeating pattern of compressions and rarefactions. Thus, the wavelength is commonly measured as the distance from one compression to the next adjacent compression or the distance from one rarefaction to the next adjacent rarefaction.
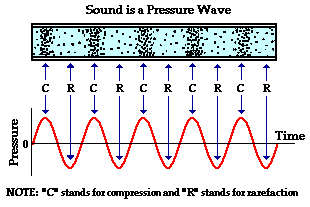
Since a sound wave consists of a repeating pattern of high-pressure and low-pressure regions moving through a medium, it is sometimes referred to as a pressure wave.
Transmission of sound
To produce vibrations that become sounds, a mechanical device (the source) must first receive an input of energy. Next, the device must be in contact with a medium that will receive the sound energy and carry it to a receiver. If the device is not in contact with a medium, the energy will not be transferred to a receiver, and there will be no sound.
Thus, three basic elements for transmission and reception of sound must be present before a sound can be produced.
They are
(1) the source (or transmitter),
(2) a medium for carrying the sound (air, water, metal, etc.), and
(3) the detector (or receiver).
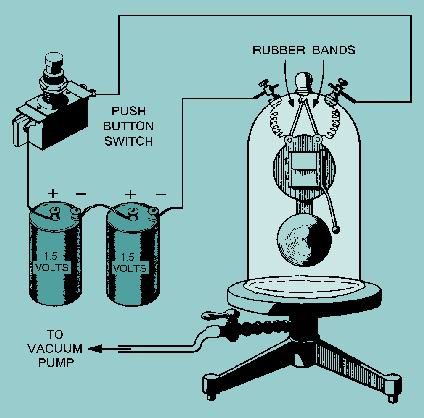
A simple experiment provides convincing evidence that a medium must be present if sound is to be transferred. An electric bell is suspended by rubber bands in a bell jar from which the air can be removed.
An external switch is connected from a battery to the bell so the bell may be rung intermittently. As the air is pumped out, the sound from the bell becomes weaker and weaker. If a perfect vacuum could be obtained, and if no sound were conducted out of the jar by the rubber bands, the sound from the bell would be completely inaudible.
Observation:
You could see the bell being struck, but you could hear no sound because there was no medium to transmit sound from the bell to you.Sound could not be transmitted through a vacuum.When the air is admitted again, the sound is as loud as it was at the beginning.
Results:
This experiment shows that when air is in contact with the vibrating bell, it carries energy to the walls of the jar, which in turn are set in vibration. Thus, the energy passes into the air outside of the jar and then on to the ear of the observer. This experiment illustrates that sound cannot exist in empty space (or a vacuum). Hence, any medium which has particles that can vibrate will transmit sound.
Now let's look at another example in which the third element, the detector, is missing. You see a source (such as an explosion) apparently producing a sound, and you know the medium (air) is present, but you are too far away to hear the noise. Thus, as far as you are concerned, there is no detector and, therefore, no sound. We must assume, then, that sound can exist only when a source transmits sound through a medium, which passes it to a detector. Therefore, in the absence of any one of the basic elements (source, medium, detector) there can be NO sound.
Measuring Speed of Sound
Speed of sound varies according the type of medium it travels through.
Sound travels fastest in solids and slowest in gases.
Equipment needed to measure the speed of soundYou will need the following items in order to measure the speed of sound:
1 50M tape measure (50 metres is very long so if you haven't got one, make a measure by getting a ball of string and measuring out 50 one metre sections. Mark each one with a bit of coloured tape.)
1 stopwatch
1 big flat outdoor wall that produces a good echo
1 friend
Measure the speed of sound1) Use the tape to measure a distance of 50 metres from the wall.
2) Now clap your hands and check you can clearly hear an echo from the wall. Make sure the echo isn't coming from other walls in the area. The time it takes sound to run 100 metres is the time difference between when you clap and when you hear the echo.

3) However measuring that single short burst of time is difficult.
4) Now clap repeatedly in time with the echo, so that you can only hear your own clap (the echo is masked by your next clap)
5) So we can now measure the time it takes to clap 10 times. Start the stopwatch at the first clap and end it when you hear the echo of the 10th clap.
6) Find the Average value, that is the average speed of sound.
(We now know how long it takes for sound to travel 1 kilometre. A little bit of maths will let us convert that into metres per hour or miles per hour.)